| RxKinetics WebApp Formulas
|
Antibiotic Kinetics©
Lean body weight (LBW)
Devine adult method1
LBW = 45.5 + [ 2.3 x (60 - Height in inches) ]
Add 4.5 kg for males
Body surface area (BSA)
Mosteller equation2
BSA = (HT X WT)2 / 3600
where
HT = height in centimeters
WT = weight in kilograms
Creatinine clearance (CLcr)
In patients 65 years or older who have a serum creatinine less than 0.8, the SrCr is rounded up to 0.8.
Cockroft and Gault method3
CLcr = [Weight x (140 - Age)] / (SCr x 72)
Decrease by 15% for females
Weight may be one of the following:
Lean body weight
Adjusted body weight
Total body weight
Adjusted body weight for obesity18
ABW = LBW + [CF x (TBW - LBW)]
CF = 0.2, 0.3, or 0.4 (user selected)
Jelliffe multi-step method5
1. Estimate urinary creatinine excretion rate (E)
E Males = LBW x [29.305 - (0.203 x Age)]
E Females = LBW x [25.3 - (0.18 x Age)]
where
LBW = lean body weight in kilograms
2. Correct E for nonrenal creatinine excretion in chronic renal failure
E = E x [1.035 - (0.0377 x SCr)]
where
SCr is the latest serum creatinine
OR if SCr is rising, the average SCr
3. Correct E for rising serum creatinine
E = E - [4 x LBW x (SCr1 - SCr2)] / D
where
SCr1 = the latest serum creatinine
SCr2 = the earlier serum creatinine
D = the number of days between
4. Calculate CLcr
CLcr = (E x 0.12) / (SrCr x BSA)
where
BSA = Body surface area
Jelliffe 1973 equation9
CLcr = 98 - [0.8 x (Age - 20)] / SrCr
Decrease by 10% for females
MDRD method6,7
CLcr = exp{ 5.228 - [ 1.154 x log(SCr) ] - [ 0.203 x log(Age) ] }
Decrease by 25.8% for females
Increase by 121% for African Americans
Normalizled CrCl method18
CLcr = (140 - Age) / SrCr
Decrease by 15% for females
Salazar and Corcoran method4
CLcr Male= { [137 - Age] x [ (0.285 x Wt) + (12.1 x Ht2) ] } / (51 x SCr)
CLcr Female= { [146 - Age] x [ (0.287 x Wt) + (9.74 x Ht2) ] } / (60 x SCr)
Swartz pediatric method8
CLcr = (c x Ht) / Scr
where
Ht = height in cm
SCr = most recent serum creatinine
c = 0.45 if age < 1 year
c = 0.55 if age 1-12 years
Prospective population model
Wagner linear method11
Kel = Knr + (Kr x CLcr)
Vd = Vdper x WtKg
where
Kel = total elimination rate
Knr = nonrenal elimination rate constant
Kr = renal elimination rate
Vd = apparent volume of distribution in liters
Vdper = population average Vd in liters per kg
WtKg = Weight in kg (may be modified for obesity)
Ideal dose calculation
One compartment intermittent infusion11
tau = tinf + [ (-1 / Kel) x ln (Cpmin / Cpmax)]
Dose = Kel x Vd x Cpmax x tinf x (1 - e-Kel x tau) / 1-e-Kel x tinf)
where
tau = dosage interval in hours
tinf = infusion time in hours
Kel = elimination rate constant
Cpmax= target peak serum level
Cpmin= target trough serum level
Dose = dose in mg
Vd = volume of distribution in liters
Serum level prediction
One compartment intermittent infusion11
Peak = [Dose / (tinf x Vd x Kel)] x [(1 - e-Kel x tinf) / (1 - e-Kel x tau)]
Trough = Peak x e-Kel x (tau-tinf)
where
Dose = chosen dose in mg
tau = chosen dosage interval in hours
tinf = infusion time in hours
Vd = volume of distribution in liters
Kel = elimination rate constant
Serum level evaluation
Standard Sawchuk-Zaske Method12
3 or 4 non-steady-state measurements
Kel = [ln (Cp2 / Cp4)] / tdiff
Vd = [(Dose / tinf) x (1 - e-Kel x tinf)] / {Kel x [Cp2 - (Cp1 x e-kel x tinf) ] }
where
Cp1= trough serum level measured prior to infusion
Cp2= peak serum level measured after the infusion
Cp3= mid-point serum level measured after the infusion (optional)
Cp4= trough serum level measured after the infusion
tdiff = time between levels in hours
tau = dosage interval in hours
tinf = infusion time in hours
Dose = dose in mg
Exception: Linear least squares determination of Kel utilized
if 3 post dose levels measured.
Steady-state Sawchuk-Zaske Method13
2 steady-state measurements
Kel = [ln (Cptr / Cppk)] / (tau - tinf)
Vd = [(Dose / tinf) x (1 - e-Kel x tinf)] / {Kel x [Cppk - (Cptr x e-kel x tinf) ] }
where
Cppk = peak serum level measured after the infusion
Cptr = trough serum level measured before the infusion
tau = dosage interval in hours
tinf = infusion time in hours
Dose = dose in mg
First dose Sawchuk-Zaske Method13
2 or 3 levels drawn after the first dose (no prior drug on board)
Kel = [ln (Cp1 / Cp3)] / tdiff
Vd = [ (Dose / tinf) x (1 - e-Kel x tinf) ] / (Cp1 / e-Kel x t1)
where
Cp1= peak serum level measured after the infusion
Cp2= mid-point serum level measured after the infusion (optional)
Cp3= trough serum level measured after the infusion
tdiff = time between levels in hours
t1 = time after infusion peak level drawn in hours
tinf = infusion time in hours
Dose = dose in mg
Exception: Linear least squares determination of Kel utilized
if 3 post dose levels measured.
Linear least squares determination of Kel14
Utilized if 3 post-dose levels measured
Kel (slope) = [(n * Sxy) - (Sx * Sy)] / [(n * Sxsq) - Sx2]
where
n = number of points
x = hours post infusion
y = natural log of measured serum level
Sx = SUM of x values
Sy = SUM of y values
Sxy = SUM of products (x * y)
Sxsq = SUM of the squares of x values
Bayesian analysis15
The Bayesian method uses population-derived pharmacokinetic parameters (ie., Vd and Kel)
as a starting point and then adjusts those parameters based on the serum level results
taking into consideration the variability of the population-derived parameters and the
variability of the drug assay procedure. To achieve that end, the least squares method
based on the Bayesian algorithm estimates the parameters Kel & Vd which minimize the
following function:
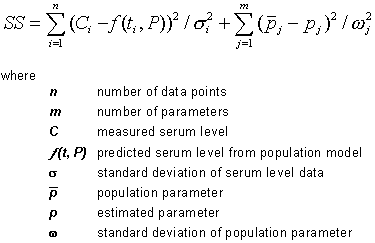
TPNassist©
Ideal body weight (IBW)
ASPEN method10
Pediatric
IBW = (HtCm2 x 1.76) / 1000
Adult
IBW Male= 48 + (2.7 x (HtIn - 60))
IBW Female= 45 + (2.3 x (HtIn - 60))
where
HtCm = height in cm
HtIn = height in inches
Nutritional dosing weight (NDW)16
If total weight (TBW) > 120% of IBW:
NDW = IBW + (0.25 * (TBW - IBW))
Otherwise:
NDW = TBW
Basal energy expenditure (BEE)
ASPEN method10
Age 1 - 2 years
BEE Male= (60.9 x Weight) - 54
BEE Female= (61 x Weight) - 51
Age 3 - 12 years
BEE Male= (22.7 x Weight) + 495
BEE Female= (22.5 x Weight) + 499
Adult
BEE Male= 66 + (13.7 x Weight) + (5 x Height) - (6.8 x Age)
BEE Female= 665 + (9.6 x Weight) + (1.7 x Height) - (4.7 x Age)
where
Height = height in cm
Weight = weight in kg
Weight may be one of the following:
Total body weight
Ideal body weight
Nutritional dosing weight
Body mass index (BMI)
BMI = WtKg / HtM2
where
WtKg = weight in kg
HtM = height in meters
Nitrogen to Noprotein calorie Ratio (N:NP)
N:NP = kCal / (Protein / 6.25)
where
kCal = total nonprotein calories
Protein = total protein in grams
6.25 g protein = 1 g Nitrogen
Ca:Phos solubility product (Ca:Phos)
Ca:Phos = Ca x Phos
where
Ca = total calcium concentration in mEq/L
Phos = total phosphate concentration in mMol/L
TPN osmolarity10
Osmol = (AA + Dextrose + Fat + Calcium + OtherLytes) / Volume
where
Osmol = approximate osmolarity in mOsmol/L
AA (amino acids) = 10 mOsmol/g
Dextrose = 4 mOsmol/g
Fat = 1.7 mOsmol/g
Calcium = 1.5 mOsmol/mEq
Other electrolytes = 1 mOsmol/mEq
Volume = Total base volume in liters
Note: does not include volume of micronutrient additives.
References
- Devine Ben. Gentamicin therapy. Drug Intell Clin Pharm 1974;8:650-6.
- Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med 1987 Oct 22;317(17):1098 (letter)
- Cockroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
- Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988 Jun;84(6):1053-60.
- Jelliffe RW, Jelliffe SM. Estimation of creatinine clearance in patients with unstable renal function. (revised). Originally published: A computer program for estimation of creatinine clearance from unstable serum creatinine concentration. Math Biosci. 14:17-24 (June) 1972.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461-470, 1999
- Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11:A0828, 2000 (abstr)
- Schwartz, GL. A simple estimate of glomerular filtration rate in children Pediatrics 1976:58:259-263.
- Jelliffe RW. Creatinine clearance: Bedside estimate. Ann Inter Med. 1973; 79:604.
- Gottschlich, MM (Ed). The Science and Practice of Nutritional Support. Dubuque: Kendall/Hunt Publishing, 2001.
- Wagner J.G. Fundamentals of Clinical Pharmacokinetics. Hamilton: Drug Intelligence Publications, 1975.
- Sawchuk RJ, Zaske DE, et al. Kinetic model for gentamicin dosing. Clin Pharmacol Ther 1977;21;3:362-369.
- Bauer, LA. Applied Clinical Pharmacokinetics. New York: McGraw-Hill, 2001.
- Bulmer MG. Principles of Statistics. New York: Dover Publications. 1967.
- Okamoto MP, Chi A, et al. Comparison of two microcomputer Bayesian pharmacokinetic programs for predicting serum gentamicin concentrations. Clin Pharm 1990 9:708-11.
- Krenitsky, J. Evidence to Support the Use of Adjusted Body Weight in Calculating Calorie Requirements. Nutrition in Clinical Practice 2005;20;4:468-473.
- Rombeau JL, Rolandeli RH. Clinical Nutrition: Parenteral Nutrition, 3rd ed. Philadelphia: W.B. Saunders Company, 2001. p. 129.
- Winter MA, Guhr KN, Berg, GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy 2012;32(7):604-612.
Donations welcome
If you find the RxKinetics WebApps to be useful in your practice, please donate to ensure their continued availability:
©Copyright 1984 - 2025, RxKinetics. All rights reserved